 Dec 6: A bulging stomach is a problem area for most mortals. Even those who are naturally thin tend to develop a paunch as they step into their 30s.
Dec 6: A bulging stomach is a problem area for most mortals. Even those who are naturally thin tend to develop a paunch as they step into their 30s.
Our stomachs store fat for a number of reasons; these reasons range from the genetic to plain abuse of food and drink, with little to no exercise. Often, those who invest heavily in exercise and diet to banish the bulging stomach, do so with a vague and incorrect idea of what is needed for a flat stomach. Today, we give you a few exercises that will strengthen and create lean abdominal muscles, help you eliminate a flabby belly and give you a flat stomach that helps you fight disease and ill-health.
The key to a flat stomach is combination
To kick that tummy fat, simply belting away crunches or pushups is not enough. A solo act can't lead to a flat tummy or fat loss. Fitness expert, Sophia Yasmin says, "In my opinion spot reduction is not possible, there is no way to target a particular part of the body for fat loss."
Your goal should be to build muscle, and focus on fat loss. Whether you are able to drop weight before attempting muscle toning, or tone muscle and then cut fat, depends entirely on how overweight you are, and how many inches you need to lose.
Follow patterned full body exercises like skipping and running to burn energy at an elevated heartrate. The kind of food you eat also helps to cut down that visible and visceral fat. A balanced healthy diet is essential for stomach fat loss.
Drink plenty of water and stay off from stress and anxiety and limit your salt intake. Besides get enough sleep to kick your belly fat.
Mentioned ahead are exercises that will help get a flat stomach.
Note: These exercises will only help you get rid of stomach fat if you practise them in combination with a healthy lifestyle and balanced diet.
Funky standing abs
This is one of the best and easiest exercises to begin with. Stand with your feet below your shoulders, and then tighten your abs slowly bending your knees.
Tilt your pelvis forward, so that your back is curved. Come back to the centre and tilt pelvis backward. Perform this exercise 15 times on either side, or as your workout permits.
Chair leg lifts
Perform this exercise using any kind of chair.
All you have to do is, sit straight with your back flat against the chair, place your hands on the seat of your chair, then slowly lift your knees towards your chest and slowly restore them back.
Carry out slow breathing while doing this. Perform this 2 set exercise at least 10-15 times.
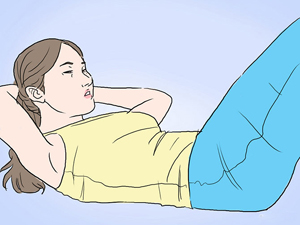
Crunches
This is a good exercise for upper, lower and oblique abdominal muscles. Begin by lying flat on the ground, with your feet placed firmly on the ground, clasp your hands behind your head.
Raise your upper body by squeezing your abdominal muscles and when you are halfway through, hold on for 3 seconds.
Then bring back your body back to floor, slowly. Do at least 30 crunches per set.
Perpendicular exercise
Lie flat on your back, with your hands behind your back. Breathe out, as you lift your legs over your hips so they are perpendicular to the floor; slightly extend the distance between your legs.
Breathe in as you lower down your legs. Start up with 4 to 5 sets, and then increase it to 10.
Dumbbell bends
This exercise is useful for your oblique muscles. Start with grabbing a dumbbell, holding it in your right hand; see to it that your palm is facing your body.
Your feet should be at a shoulder-width distance. Slowly place your left hand on your hip and bend your upper body towards the right, while keeping your head and body facing forward.
Bring back your body to normal position and then repeat the same movement on the left side. Practice 20 repetitions.
Bicycle exercise
Bicycle is the best exercise for toning your stomach. It helps by keeping your stomach stable, along with movements, which burns fat.
Perform this exercise by lying on the floor, place your hands behind your head and bring your knees off the floor.
Bring your right elbow towards your left knee while performing cycling motion, and then switch your elbow position.
Side exercise
This exercise targets your oblique, core muscles and shoulders. Stand straight, with your feet at approximately the width of your hips. Slowly bend your knees and hold dumbbells in each hand.
Lift your hands up, so that the dumbbells are above your head and relax. Then lean your arms, head and torso to the right till 2 counts, then come back to the original position and then repeat it to the left side.
Carry out at least 10 repetitions.
Planks
Lie on the floor, with your face down, upper body supported on your forearms. Raise your entire body off the floor, with the support of your forearms and toes form a straight line.
Carry out 3 repetitions, with 15 to 20 seconds hold.
Clock exercise
To carry out this exercise, you may need an exercise ball. Rest your back on the ball with your feet aligned with your hips.
Stretch your arms over your head, contracting your abdominal muscles, and then rotate your body like a clock. Carry out 10 rotations in each side.





Comments
Add new comment