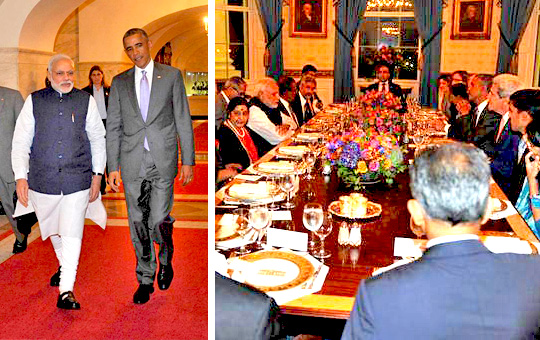
 Washington, Sep 30: Ahead of their Summit talks, US President Barack Obama today hosted private dinner for Prime Minister Narendra Modi as the two leaders sought to reinvigorate bilateral ties.
Washington, Sep 30: Ahead of their Summit talks, US President Barack Obama today hosted private dinner for Prime Minister Narendra Modi as the two leaders sought to reinvigorate bilateral ties.
The Prime Minister, who arrived here late afternoon at the Andrew Airforce Base, was received by William Burns, Deputy Secretary of State and other senior officials.
From there, Modi left for the Blair house, the American President's guest house where he will be staying during his Washington trip. Later, he drove to the White House from the Blair guest house for the dinner.
"Kem Cho," asked Obama when he welcomed Modi, who replied, "Thank you very much, President."
The dinner, with limited guests from each side in the Blue Room of the White House might have a delectable spread of dishes, but the main guest only had warm water as he was observing Navratri fasts.
However, First Lady Michelle Obama did not attend the dinner as she was travelling.
"The Prime Minister did not had anything except for warm water," External Affairs Ministry Spokesperson Syed Akabruddin said.
Later, Modi said he had a wonderful meeting with Obama during which they talked about a wide range of issues.
"Obama & I share a vision for a partnership in which our nations work together for the benefit of the entire humankind," the Prime Minister tweeted.
On the issues of discussion during the 90-minute dinner meeting, Akabruddin said discussions were largely on knowing each other and sharing the initial experiences after they took over.
They shared anecdotes to connect with each other, he said while describing the dinner meeting as a "very successful interaction".
"They did not get to discuss any of the substantive issues. This was a very cordial and comforting conversation where each of them were trying to understand others perspective and they did not get into very substantive discussion which will follow tomorrow," he added.
They agreed to take up more substantive issue tomorrow -- both in restrictive format and in delegation level talks -- but in general the thinking was that there was a lot of goodwill between India and the US for each other, he said.
"There was a feeling that they should try and focus on some big things that they can achieve in a finite time period in the next few years," the Spokesperson said.
"There was also a feeling that India-US relationship was among the most important relationships in the world today. And therefore, it was incumbent upon both of them to work towards strengthening and deepening this relationship," he said.
The Prime Minister was asked to outline his vision of what were the issues he faced so far and how he was working on it, the Spokesperson said.
"Remarkably some of those things the Prime Minister mentioned seemed to have resonance with President Obama, because he said he had similar concerns when he came in power.
Giving example of how the two leaders connected, he said Prime Minister told Obama that when he came to Delhi he found that the technological infrastructure in the Indian capital was not even as good as Gujarat to which the US President said he had similar experience.
They both talked about their focus areas of technology and e-governance.
The two leaders have jointly written an editorial in a US newspaper which would be published tomorrow.
Modi also laid out in great details his hopes and aspirations in terms of what was his development vision and how the US can help in achieving that. There was general understanding that the US and India can work not only in terms of bilateral relations but elsewhere in the world, the Spokesperson said, adding in this context they talked about Ebola disease and Afghanistan. These were the two issues that were broadly flagged, he said.
Basically, today's meeting had three major components -connecting with each other, vision for the ties and cooperation in various areas, the Spokesperson added.





Comments
Add new comment