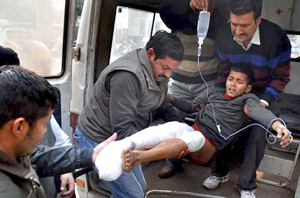
Srinagar/Jammu, Jan 3: Two army jawans and a woman were killed while 11 people were injured as Pakistani troops targeted villages and security posts at various places in Jammu and Kashmir since last night which triggered migration of hundreds of people from some border areas.
While the firing and shelling continued, Home Minister Rajnath Singh regretted that Pakistan is repeatedly targeting border areas of India despite being offered a hand of friendship.
Pakistani troops violated the ceasefire along the LoC near Kanhaiyah Post last night by firing rocket propelled grenades into Tanghdar area, police officials said while citing the FIR lodged by the army.
They said the Pakistani action caused death of two army jawans and injury to another. A BSF jawan, who was posted on duty in a nearby post of the force, was also injured in the incident, the officials said.
Along the International Border, Pakistani troops targeted villages and 13 border outposts with heavy mortar shelling in Kathua and Samba districts of Jammu and Kashmir, leading to death of a woman and injuries to eight others.
Shelling of villages triggered panic and migration from border villages and over 1400 people have been evacuated from hamlets in Samba and Kathua district, officials said.
"Pakistani troops resorted to unprovoked and heavy firing and mortar shelling on posts and civilian areas along IB in Samba and Kathua districts since 2130 hours last night," Inspector General of BSF Rakesh Sharma told PTI.
"BSF troops gave a befitting reply resulting in exchanges which stopped at 3 AM in the morning," the IG said.
Pakistan again started mortar shelling and firing targeting civilian areas since 7 AM, he said.
He said all BoPs in three battalion areas of Samba and Hiranagar sub-sectors in Kathua district were targeted.
A woman identified as Tori Devi of village Mangu Chack has been killed in the shelling and four civilians have been injured in the firing and shelling, Senior Superintendent of Police (SSP), Samba, Anil Magotra told reporters.
Four persons, including two women, were injured in Nauchak village in Kathua, Deputy Commissioner Kathua Shahid Iqbal said.
IG BSF has also issued an advisory to the border people for evacution.
Scores of houses have suffered damage and few animals have also perished in the firing, Magotra said.
The SSP said buses have been kept ready for evacuation and "we are waiting for the firing and shelling to stop so that people are shifted to safer areas in shelter camps".
People have taken shelter in bunkers and are not venturing out, he said.
The latest round of firing by Pakistan which started on New Year eve has left two persons dead, including a BSF jawan, and nine injured while five Pakistani Rangers have been killed in retaliatory firing by India.
Commenting on this, Rajnath Singh said in Delhi, "Pakistan should stop ceasefire violations."
He wondered why Pakistan was continuously indulging in ceasefire violations despite having suffered badly every time.
"While we are offering our hand of friendship to Pakistan, it is continuously indulging in ceasefire violations. We made a beginning by inviting Pakistan Prime Minister Nawaz Sharif at the swearing-in of Prime Minister Narendra Modi, who shook hands with him offering not just friendship but also hoping to unite hearts. Despite that, Pakistan is indulging in ceasefire violations repeatedly," he said.
He said India wants good ties with all our neighbouring countries, including Pakistan.
The renewed ceasefire violations come barely two months after the last major escalation that left 13 people dead and displaced 32,000 border residents in August and October.
Over 550 incidents of ceasefire violations by Pakistan occurred in 2014, the highest since the truce came into force in 2003, with the Indo-Pak border witnessing the worst such escalation during August to October which left 13 people, including 2 security personnel dead.
A total of 19 people, including 5 jawans, were killed and over 150 injured in such incidents last year.





Comments
Add new comment