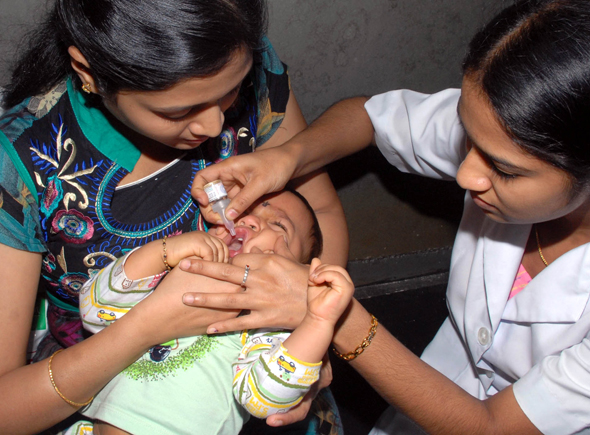
 Bangalore, Feb 25: Nearly 10 lakh of the 74.31 lakh children below the age of five in the State are yet to be administered oral polio drops during the second round of the Pulse Polio immunisation drive that began on Sunday.
Bangalore, Feb 25: Nearly 10 lakh of the 74.31 lakh children below the age of five in the State are yet to be administered oral polio drops during the second round of the Pulse Polio immunisation drive that began on Sunday.
By covering more than 64.46 lakh children across the State on day one of the drive, the Health Department has claimed to have achieved 86.75 per cent coverage. The remaining children will be covered in the next three days when the health department officials and volunteers will go on a door-to-door campaign to ensure that all the children are administered polio drops
While districts such as Udupi, Kodagu, Tumkur, Shimoga and Mysore achieved over 90 per cent coverage, the coverage was less than 80 per cent in north Karnataka districts such as Bidar, Gulbarga and Yadgir.
Speaking to The Hindu, B.V. Karur, Project Director, Reproductive and Child Health programme said, “I appeal to the people to cooperate and get oral polio drops administered for their children during the house to house visits.”
Meanwhile, in Bangalore, more than 1.13 lakh children are yet to be immunised in the second round conducted the Bruhat Bangalore Mahanagara Palike (BBMP) limits. Around 5.36 lakh children were given drops on Sunday achieving 82.49 percent coverage.
Devaki Umesh, Chief Health Officer, BBMP said, “We will try to achieve our target during our door-to-door visits and make special preparations to ensure that we provide drops to the high risk groups such as migrants.”
Meanwhile, when The Hindu visited some of the pulse polio booths in the city, a few parents complained that they had to travel a longer distance this time to get the polio drops administered to their children.
Sayeeda Khurram, a two-year old, was brought to the booth at Yelahanka General Hospital by her mother from Kogilu. Her mother said, “Last time it was easy for us to get these drops as a booth had been set up near our house.”
Harishith G., had come to Rajajinagar Maternity Home to get polio drops administered for his three-year-old relative.
He said, “There has been wide publicity for this campaign. All vaccinations should receive coverage like this one.”





Comments
Add new comment