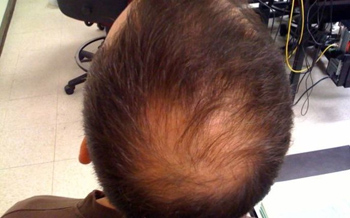
 Washington, Jun 21: In a first, scientists have used an arthritis drug to successfully grow a full head of hair on a 25-year-old man suffering from a non-curable disease that left him without any hair on the body. After the treatment, the man grew a full head of hair, eyebrows, eyelashes, armpit, facial and other hair on his body. There is currently no cure or long-term treatment for alopecia universalis, the disease that left the patient hairless, researchers said. This is the first reported case of a successful targeted treatment for the rare, highly visible disease. “The results are exactly what we hoped for,” said Brett A King, assistant professor of dermatology at Yale University School of Medicine and senior author of the research paper.
Washington, Jun 21: In a first, scientists have used an arthritis drug to successfully grow a full head of hair on a 25-year-old man suffering from a non-curable disease that left him without any hair on the body. After the treatment, the man grew a full head of hair, eyebrows, eyelashes, armpit, facial and other hair on his body. There is currently no cure or long-term treatment for alopecia universalis, the disease that left the patient hairless, researchers said. This is the first reported case of a successful targeted treatment for the rare, highly visible disease. “The results are exactly what we hoped for,” said Brett A King, assistant professor of dermatology at Yale University School of Medicine and senior author of the research paper.
Vitamin E may harm, or help, your lungs
New York: A form of vitamin E found in vegetable oils like corn and canola may worsen lung function, while another form typically found in olive oil may protect it, a new study suggests. The findings may help explain why studies of the health effects of the vitamin have had conflicting results. Vitamin E comes in various forms called tocopherols, which are commonly found in fats and oils. Supplements of the vitamin may contain a single type of tocopherol, or a mix. The new research, published in the journal Respiratory Research, found that a form of the vitamin called gamma tocopherol, the kind in corn, canola and soybean oils, was linked to poor lung function in adults. But another form of the vitamin more typically found in olive and sunflower oils, called alpha tocopherol, seemed to have a beneficial effect on the lungs.





Comments
Add new comment