New Delhi, May 12: Two eminent scholars have resigned their positions as advisers to the National Council of Educational Research and Training (NCERT) after a furore in Parliament led Human Resource Development Minister Kapil Sibal to withdraw a book on the Constitution because it contained a cartoon some legislators said was offensive.
Yogendra Yadav and Suhas Palshikar, both eminent political scientists, resigned hours after the cartoon provoked a furore in Parliament.
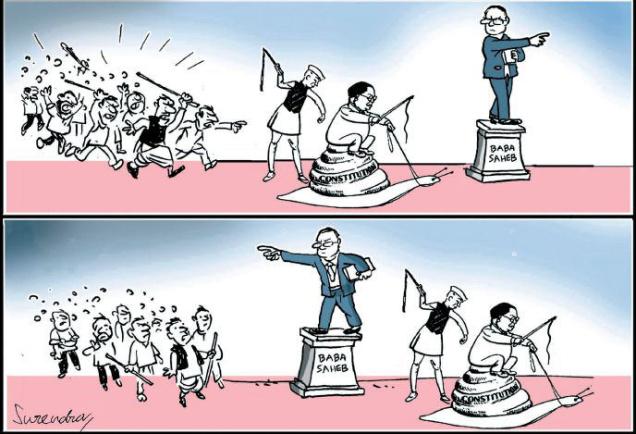
The 11th-class textbook, Indian Constitution at Work, includes a cartoon which shows Dr. B.R. Ambedkar sitting on a snail, a whip in hand, while Prime Minister Jawaharlal Nehru stands behind him, also armed with a whip.
A caption accompanying the drawing, which was made by the legendary cartoonist K. Shankar Pillai in 1949, explains that it illustrated “the ‘snail's pace' with which the Constitution was made.”
Dr. Yadav said: “The heated and not very well-informed debate in Parliament did not do justice to the responsibility that a democratic society has towards future generations.” He pointed out that the decision implied “a law must be passed to ban all cartoons.” Dr. Palshikar was not available for comment.
Shankar regularly drew cartoons for his weekly, Shankar's Weekly. His depiction of Ambedkar and Nehru triggered no controversy at the time and his public service was recognised by successive governments which awarded him the Padma Shri, Padma Bhusan and Padma Vibhusan.
Furore in Parliament
Responding to the furore in both Houses, Mr. Sibal said he had “directed the NCERT to stop the distribution of these textbooks. A committee had been set up to review not just the cartoons but the content of these textbooks as well.” “For the next year,” Mr. Sibal assured MPs, “we will remove all these cartoons. But even this year, till we review the situation, the present textbooks will not be distributed.”
The government would not allow Dr. Ambedkar's memory to be “disparaged.” “When I got information [on this issue] in the beginning of April,” he said, “I wrote a letter to NCERT that such a cartoon should not be there as it is objectionable.” The government would also examine whether those who drew the allegedly objectionable cartoon had committed a criminal offence. Mr. Pillai died in 1989.
Earlier, Viduthalai Chiruthaigal Katchi member Thol. Thirumavalavan, who raised the issue in the Lok Sabha, said the cartoon had insulted both Nehru and Dr. Ambedkar. He sought Mr. Sibal's resignation and withdrawal of the book. In the Rajya Sabha, BSP leader Mayawati sought criminal action against those responsible.
Lok Jan Shakti Party leader Ram Vilas Paswan sought dismissal of those responsible for allowing the cartoon to be published.
Finance Minister Pranab Mukherjee described Dr. Ambedkar as the Ved Vyas of the Constitution, and said the cartoon was “totally wrong.”
D. Raja (CPI) said the matter was “serious” as tension prevailed in Tamil Nadu after this incident came to light.
The AIADMK, the CPI, the CPI(M), the SP, the RJD, the LJP, the BJP, the TDP and the BSP also joined in the attack on the textbook.





Comments
Add new comment